
iBiotec
Formulator,
Manufacturer of technical solvents

iBiotec offers an extensive range of alternative solvents with no hazard symbols
substitutes for harmful, irritating, hazardous CMR
or highly flammable solvents
iBiotec is deeply committed to developing technologies that contribute to reducing risks for both humans and the environment. This commitment is reflected in the product ranges iBiotec offers its customers, as well as in the management policy of our site and of the customer's manufacturing site.
Our laboratories are staffed and equipped to be highly responsive to our customers' needs; which also feeds into our policy of regularly launching innovative products.
To support our internal resources, iBiotec maintains a network of strong relationships with major external laboratories, scientific committees, universities, and international suppliers via targeted partnerships.
iBiotec also participates actively, with its customers, in order to decrease the number of hazard symbols and warning phrases, as defined by CLP and GHS regulations, on its products.
This risk-reduction approach is part of a continuous improvement process in accordance with
ISO 9001
ISO 14 001
OHSAS 18 001
ISO 14 040
WCM World Class Manufacturing
LEAN manufacturing TIE elimination of MUDAs
ISO 26000
iBiotec FACTS & FIGURES
Effective and experienced staff: 38
Including 12 managers
Average years of experience: 21
Production area: 17,900 m2
Ultra-clean storage area: 20,000 m2
Research and development laboratories: 1,700 m2
Production: Manufacturing units: 8
6,000 to 14,000 Litres/hour, cold mixer, hot mixer, reactor.
Packaging: Aerosols
Three fully automated lines: Pamasol Rotary
Automatic installation of valves, nozzles, caps, weight checks. PLC-controlled
Capacity 60 cans/minute per line
PREDICTIVE MAINTENANCE = 0 FAULTS in 10 years
Automatic decoration by silk screen printing, in upstream flow
STRATEGIC DATES
1970 Company created, iBiotec initially located in Pierrefitte (Seine-Saint-Denis)
1972 Patent filed for invention of evanescent fluids
1974 Patent filed for addition of MoS2 to a lubricant
1977 Patent filed for the replacement of sulpho-chlorinated additives
1980 Creation of a production unit at Saint-Leu-la-Forêt (Val-d'Oise)
1987 Creation of a production unit at Saint-Rémy-de-Provence (Bouches-du-Rhône)
1990 Patent for the invention of an exclusive aerosol can gassing process through
in-pack gassing combined with the gasser shaker technique, in order to use a propellant of natural origin that is inert, non-flammable, non-combustible, bacteriostatic, and of a medical, pharmaceutical and food-grade quality, in addition to being available in an unlimited supply
1992 Establishment in Wroclaw, Poland
1994 Establishment in Spain and Italy
1996 Doubling of the production unit at Saint-Rémy-de-Provence
2002 Patent for a nano lubrication invention
2008 Launch of an industrial range derived from green chemicals
2014 Establishment in Southeast Asia, Malaysia, Korea, Taiwan
2015 Establishment in the Pacific region; Australia
2015 Zero Risk formulas, with no hazard symbols, recognised CLP/GHS
2016 Launch of the first detergents derived from blue chemicals (marine algae)
2016 Launch of SUPRA LUBRICANTS based on nano-particles for micro-friction
2017 Establishment in Thailand
Production site

iBiotec®
Tec Industries®Service
Based on the principles of the following current standards:
ISO 9001 - ISO 14001 - ISO 45001
Integrated Management System
ISO 14040 - Life Cycle Assessments
ISO 26000 - Social Responsibility
Production site
Z.I La Massane
13210 Saint-Rémy-de-Provence - France
Tel.: +33 (0)4 90 92 74 70
Fax: +33 (0)4 90 92 32 32

iBiotec
Two 14,000 L/h manufacturing pilots
Two 10,000 L/h manufacturing pilots
Two 6,000 L/h manufacturing pilots
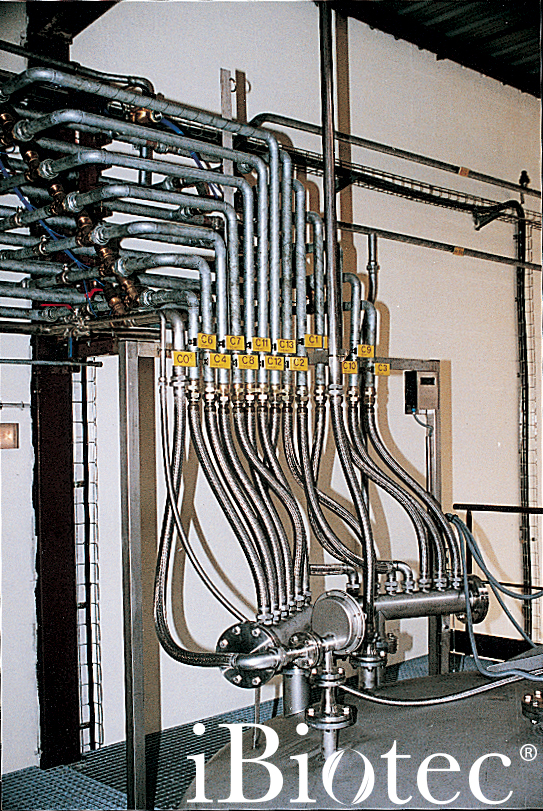
Manufacturing controlled by PLC

8,000 m2 dust-free, secure warehouses.
All our products are in stock.
Our fully-integrated production and packing process
replenishes stock levels immediately.

Raw materials storage

In accordance with the requirements and specific instructions of our Customers,
iBiotec can deliver every batch
With a certificate of compliance.
With an analysis certificate.
With an analysis report from an outside laboratory accredited by COFRAC, BPL, ISO 17.025.
With an IR spectrometry result (for products where viscosity allows this).
iBiotec ensures traceability of all batches and stores data for ten years
 |
 |

